New Drug For Heart Disease
New drug for heart disease. Another drug developed by Pfizer is further behind the approval process. A new molecule could help prevent heart failure. Statins are the mainstay drugs for heart attack prevention and work primarily by lowering cholesterol levels.
Congenital Heart Disease in Children. May 6 2020 -- The FDA has approved a new use for the drug dapagliflozin Farxiga -- to reduce the risk of a hospital stay or death in people who have a type of heart failure. The drug which goes by the name aducanumab and was made by Biogen is the first drug approved to slow the cognitive decline caused by the disease.
AstraZenecas diabetes drug Farxiga has become the first in its class to receive FDA approval as a treatment for heart failure. Among nearly 1000 people with heart disease in six countries they found that the combined effect of the PCSK9 inhibitor evolocumab and a statin lowered LDL. All three belong to a new class called PCSK9 inhibitors which work by.
For those affected calcium starts to accumulate in their heart valves and. The researchers in Brazil and the United States who developed and tested the experimental drug have named it. There is now a new medicine for heart failure patients.
One group received Corlanor while the other received a placebo. In an international clinical trial researchers divided 6500 patients into two groups. Finding new drugs for heart-valve disease An artificial intelligence-enabled drug screen finds small molecules that can correct dysfunctional gene networks in aortic valve disease.
Entresto is the first in a new class of drugs called angiotensin receptor neprilysin inhibitors or ARNIs. In April this year Corlanor became the first new chronic heart failure drug approved by the FDA in nearly a decade. But a quarter of people who have one heart attack will suffer another within five years.
On May 3 the US. Deepak Srivastava and his team found a drug candidate that could help prevent tens of thousands of heart surgeries every year.
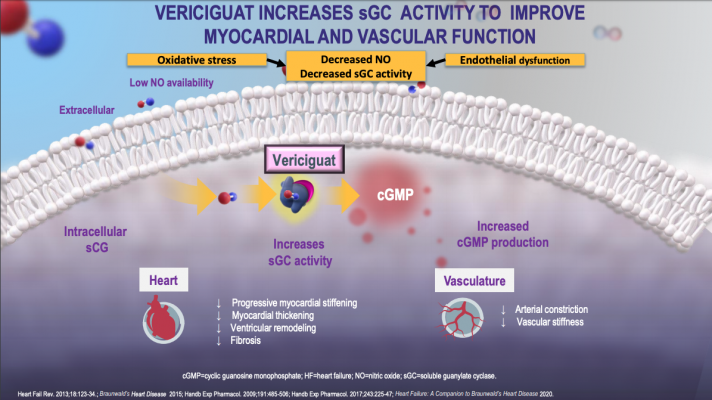
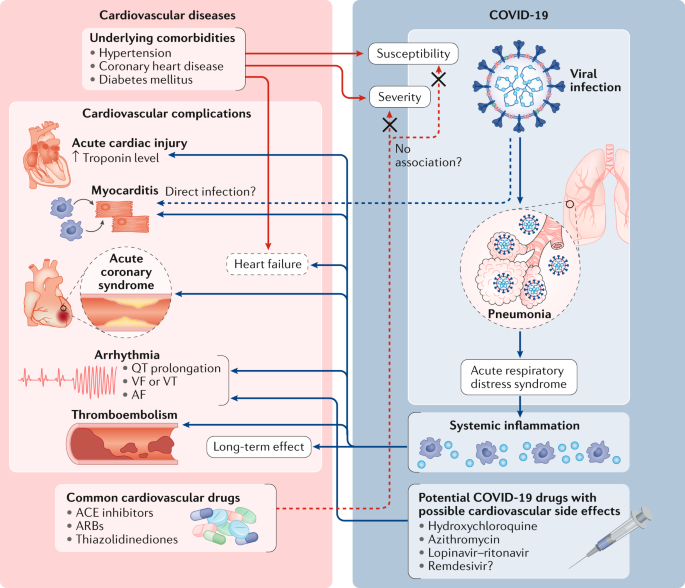
Two new agents for the treatment of chronic HFrEF LCZ696 and ivabradine have been recently been approved for use by the FDA and four novel agents which have shown considerable promise in early studies omecamtiv mecarbil vericiguat finerenone and neuregulin are currently being evaluated in late-phase clinical trials.
The drug which goes by the name aducanumab and was made by Biogen is the first drug approved to slow the cognitive decline caused by the disease. A new molecule could help prevent heart failure. Entresto is the first in a new class of drugs called angiotensin receptor neprilysin inhibitors or ARNIs. The drug which goes by the name aducanumab and was made by Biogen is the first drug approved to slow the cognitive decline caused by the disease. Among nearly 1000 people with heart disease in six countries they found that the combined effect of the PCSK9 inhibitor evolocumab and a statin lowered LDL. New heart disease drug to be made available for NHS patients The government is collaborating with pharmaceutical company Novartis to launch a clinical trial for new cholesterol. The researchers in Brazil and the United States who developed and tested the experimental drug have named it. All three belong to a new class called PCSK9 inhibitors which work by. AstraZenecas diabetes drug Farxiga has become the first in its class to receive FDA approval as a treatment for heart failure.
One group received Corlanor while the other received a placebo. New heart disease drug to be made available for NHS patients The government is collaborating with pharmaceutical company Novartis to launch a clinical trial for new cholesterol. A new molecule could help prevent heart failure. Dapagliflozin is the first and only drug under the sodium glucose transport protein 2 inhibitor SGLT2i class approved to treat heart failure in adult patients with reduced ejection fraction pumapalyang puso. There is now a new medicine for heart failure patients. Vericiguat is the first drug of its kind to be investigated as a once-daily oral treatment for patients with worsening chronic heart failure. AstraZenecas diabetes drug Farxiga has become the first in its class to receive FDA approval as a treatment for heart failure.












/overview-of-heart-disease-4160961_final-152f46073f2242999b771e409973825b.png)























Post a Comment for "New Drug For Heart Disease"